Dr. Ahmed Alrifaiy, Experimentell metodik och termodynamik - Luleå tekniska universitet
Lab on a chip for electrophysiological measurements with control of the oxygen content - optical manipulation and spectroscopic analysis of biological cells.
Time: Thu 2014-05-08 09.00 - 10.00
Location: AlbaNova FB42
Contact:
Abstract:
Stroke is a brain damages related to hypoxia, which worldwide affects 15 million people each year. To minimize suffering and costs related to the disease extensive research is carried out. Our research focuses on the fundamental understanding of the mechanism behind hypoxic brain damage and the activation of essential bio- molecular defense mechanisms which may reduce brain damage. More knowledge may hopefully lead to new therapeutic and preventive strategies at the molecular level to prevent stroke. The research focus is on hemoproteins, i.e. neuroglobin (Ngb) that mainly exists in the neurons of the brain. Earlier studies have reported the protective effect of protein against hypoxia-related brain damages. However, it is unclear how Ngb functions in the nervous system.
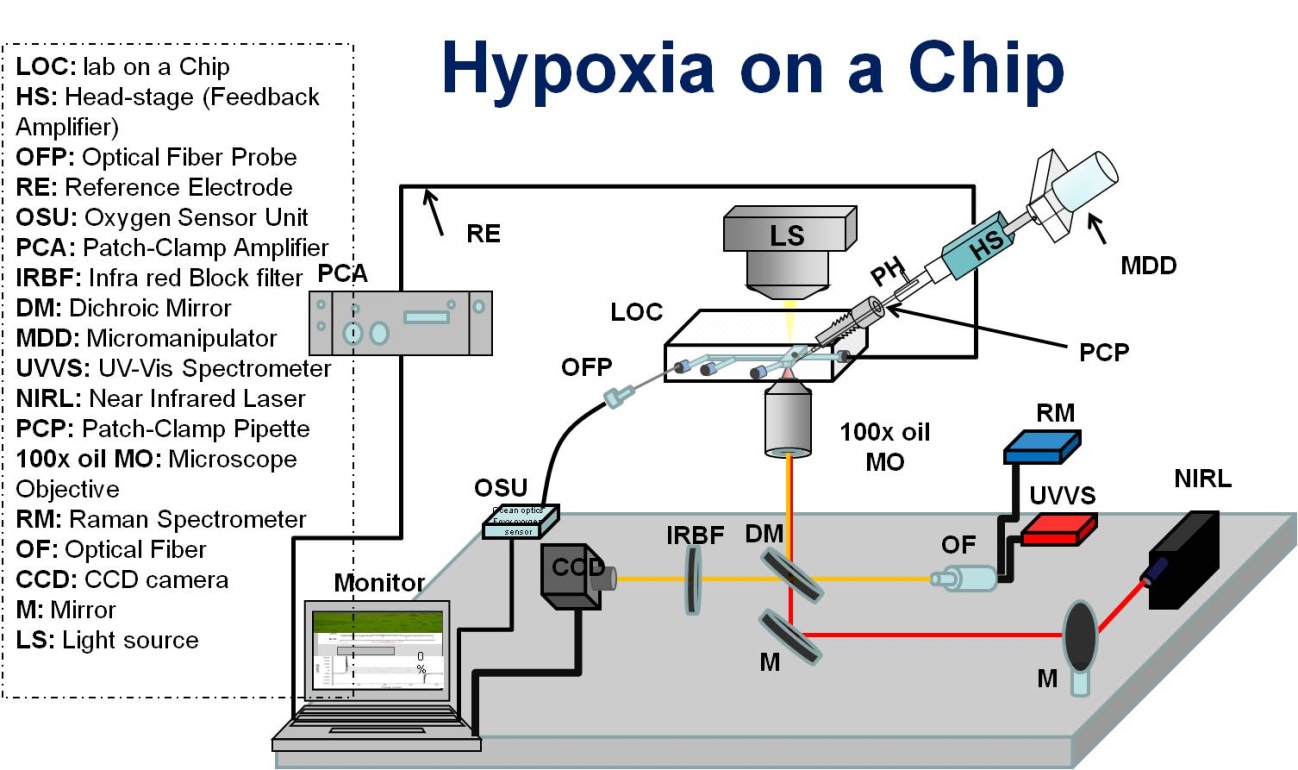
The main approach of the research was to reproduce hypoxia on a chip under similar conditions that occur within the brain. Patch-clamp technique was used to perform investigations on single nerve cells exposed to anoxic and normoxic environments. Patch-clamp is a well-established technique that is routinely used to measure and analyze the electrophysiological activity of individual biological cells. To perform accurate patch-clamp experiments, it is important to establish well-controlled physiological conditions, i.e. different oxygen levels and changes of the biochemical substances or nutrients. A promising approach is to develop and apply Lab-On-a- Chip (LOC) technologies combined with optical manipulation-, and readout technique, to give full control of rapid altering environmental conditions.
Here, the conventional open patch clamp technique, that not provides adequate control of the oxygen content, was modified to be combined with a developed gas-tight LOC device, i.e. a microfluidic system with capability to include a patch-clamp micropipette. The system was combined with an on-lab establish optical and laser systems, mainly optical tweezers and optical spectroscopy. Optical tweezers enabled the manipulation and steering of the single biological cells within the LOC device towards the fixed patch clamp micropipette. Optical spectroscopy, using NIR lasers, contributed to monitoring and investigation of the biochemical composition and state of the sample. To obtain full control over the hypoxic condition, an oxygen sensing system was incorporated within the multifunctional system in which the optical fiber was integrated within the LOC device. Thus, this multifunctional system was set up to enable simultaneous optical, electrophysiological and spectroscopic experiments with good control over the oxygen content.
The system was evaluated by proof of concept experiments. Optically trapped red blood cells and neurons were steered to the fixed patch clamp pipette within the microfluidic system. The oxygen content within the microfluidic channels was measured and verified to be less than 0.5 % compared to the usual systems 4-7%. The system enabled the microscopic monitoring of real-time optical trapping dynamics and simultaneously the spectroscopic measurements to acquire absorption spectra of the trapped cells under variations of the environments. The non-damaging effect of the optical laser-tweezers on the sample was verified experimentally. The developed multifunctional LOC system was tested for patch clamp measurements and good experimental conditions were achieved.
In order to achieve this experimental system, the gastight LOC system was designed, developed and functionally tested, as a core of multifunctional microfluidic system in which the developed techniques below will be combined.
Optical (Raman and Absorption) spectroscopy are UV-Vis and laser based techniques combined to identify Ngb 's oxidation state, i.e., if the oxygen is bound or not.
Patch Clamp technique is Nobel-priced technique used to measure the electrophysiological activity through the membrane of the biological cells under variation of environments (oxygenation states). Optical Tweezers is laser based technique using highly focused laser light to trap and move microscopic objects such as biological cells, without touching them. Laser light hits a biological cell that has
different refractive index than the surroundings; the light beam's direction and photons angular momentum are changed and thus generate trapping forces on the cell at the beam focus.
Lab on a chip (LOC) technologies provide devices associated micrometer-sized microfluidic channels, nano- and micro fluid flow, fabricated on a substrate, with multiple inputs and outputs. The biological cells are introduced into the chip via inlets, manipulated optically and investigated while the environment of the cell changes experimentally. A gastight lab on a chip provides complete control over the oxygen level of cell's environment.
Moreover, the PhD thesis presents a review on LOC devices, with focus on current advances in LOC technologies, mainly the fabrication methods, implementation and miniaturization as well as the main applications related to life science, biomedical engineering, pharmacy and cell/tissue biology.
The straightforwardness and stability of the developed LOC system have shown good potential to be used for multiple investigations that demand full control of the environments. The long term goal is to study the response of individual neurons and defense mechanisms in hypoxic conditions that may establish new ways to understand cell behavior related to Neuroglobin for various diseases such as stroke, Alzheimer's and Parkinson's.